Clinical trials are research studies conducted on humans to evaluate the safety and efficacy of new drugs, treatments, or medical procedures. They are conducted by medical professionals and researchers to collect data about the safety and effectiveness of a potential treatment or medical device. Clinical trials are a critical part of the process of bringing new treatments and therapies to market.
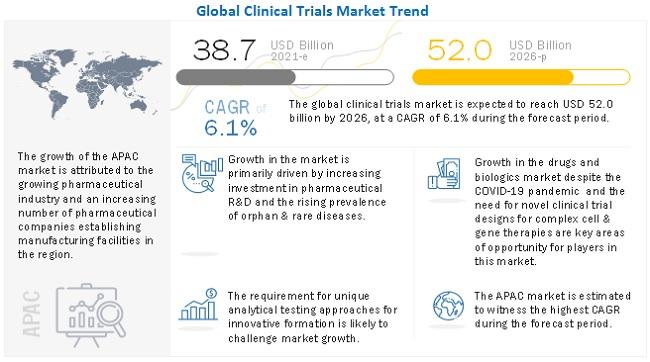
Currently, Clinical Trials Market are an integral part of the healthcare industry, providing the data and evidence needed to develop new treatments and therapies. As the demand for new treatments and therapies increases, so too does the demand for clinical trials. The global clinical trials market is expected to reach $52 billion by 2026, driven by the increasing demand for new treatments and therapies, advancements in clinical trial technologies, and the growing need for personalized medicine.
The clinical trial market is divided into two segments: clinical research organizations (CROs) and contract research organizations (CROs). CROs provide services such as clinical trial design, protocol development, patient recruitment, data management, and regulatory compliance. They also provide services such as monitoring, clinical data analysis, and report writing. Meanwhile, contract research organizations provide services such as project management, data management, and clinical data analysis. In addition, they provide services such as safety monitoring, patient recruitment, and regulatory compliance.
The growing demand for personalized medicine is driving the growth of the clinical trials market. Personalized medicine is the use of genetic and molecular data to tailor treatments to individual patients. This allows for more effective and targeted treatments, resulting in better patient outcomes. The increasing prevalence of chronic diseases is also driving the growth of the clinical trials market. Chronic diseases such as diabetes, cancer, and cardiovascular disease require new treatments and therapies, which are developed through clinical trials.
Clinical trials are an essential part of the development of new drugs, treatments, and therapies, and the clinical trials market has grown significantly in recent years. Clinical trials are conducted to evaluate the safety, efficacy, and effectiveness of new medicines, treatments, and therapies. They are a vital part of the drug development process, and are conducted in accordance with the regulatory guidelines of the relevant country.
The increasing use of digital technologies in clinical trials is also driving the growth of the market. Digital technologies allow for faster and more efficient data collection, resulting in faster and more accurate clinical trial results. In addition, digital technologies are allowing for the remote monitoring of clinical trials, making them more accessible and cost-effective.
Overall, the clinical trials market is expected to experience significant growth in the coming years. The increasing demand for personalized medicine and the use of digital technologies in clinical trials are driving the growth of the market. As the demand for new treatments and therapies continues to grow, so too will the demand for clinical trials.
Request for assumptions & how numbers were triangulated.
https://www.marketsandmarkets.com/requestsampleNew.asp?id=405
Major players in the global clinical trials market include IQVIA (US), LabCorp (US), PPD (US), PRA Health Sciences (US), Syneos Health (US), Charles River Laboratories (US), WuXi AppTec (China), Paraxel International (US), and ICON Plc (US).
The clinical trials market offers great potential for growth, as more and more companies are investing in drug development and clinical trial processes. This, in turn, is expected to drive the growth of the market over the forecast period. Additionally, the rising demand for personalized medicines, the increasing utilization of electronic data capture (EDC) systems, and the increasing number of clinical trials are also expected to contribute to the growth of the market.
What Are the Benefits of Participating in a Clinical Trial?
Participating in a clinical trial can provide several benefits to those involved. Not only can participants gain access to new treatments and medications before they become available to the general public, but they can also play an important role in advancing medical innovation. Participants also have the chance to receive personalized medical care and support, as well as financial compensation for their time and effort.
Download an Illustrative Overview:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=405
Recent Developments
- In September 2021, Syneos Health entered into a strategic collaboration with Ride Health to offer non-emergency medical transportation (NEMT) for clinical trial participants.
- In April 2021, IQVIA acquired Q2 Solutions, a clinical laboratory services organization, from Quest Diagnostics.
- In November 2020, WuXi Apptec expanded its Cell & Gene Therapy Platforms with capabilities to provide high-quality and cost-effective supplies of R&D and GMP Plasmids
- In April 2020, IQVIA launched COVID-19 trial matching service to accelerate treatment and vaccine development against the COVID-19 pandemic in U.S. The company launched comprehensive online screener and trial matching tool for all COVID-19 trials in the US.
- In May 2020, IQVIA announced the Japan and Asia Pacific expansion of IQVIA Biotech to deliver integrated clinical solutions and support biotech and emerging biopharma companies.
Content Source:
https://www.marketsandmarkets.com/Market-Reports/clinical-trials-market-405.html